Cerebellar Degeneration or CA
Canine Hereditary Ataxia in Gordon Setters – an Update December 2015
Reprinted with the permission of Dr. Natashia Olby
History
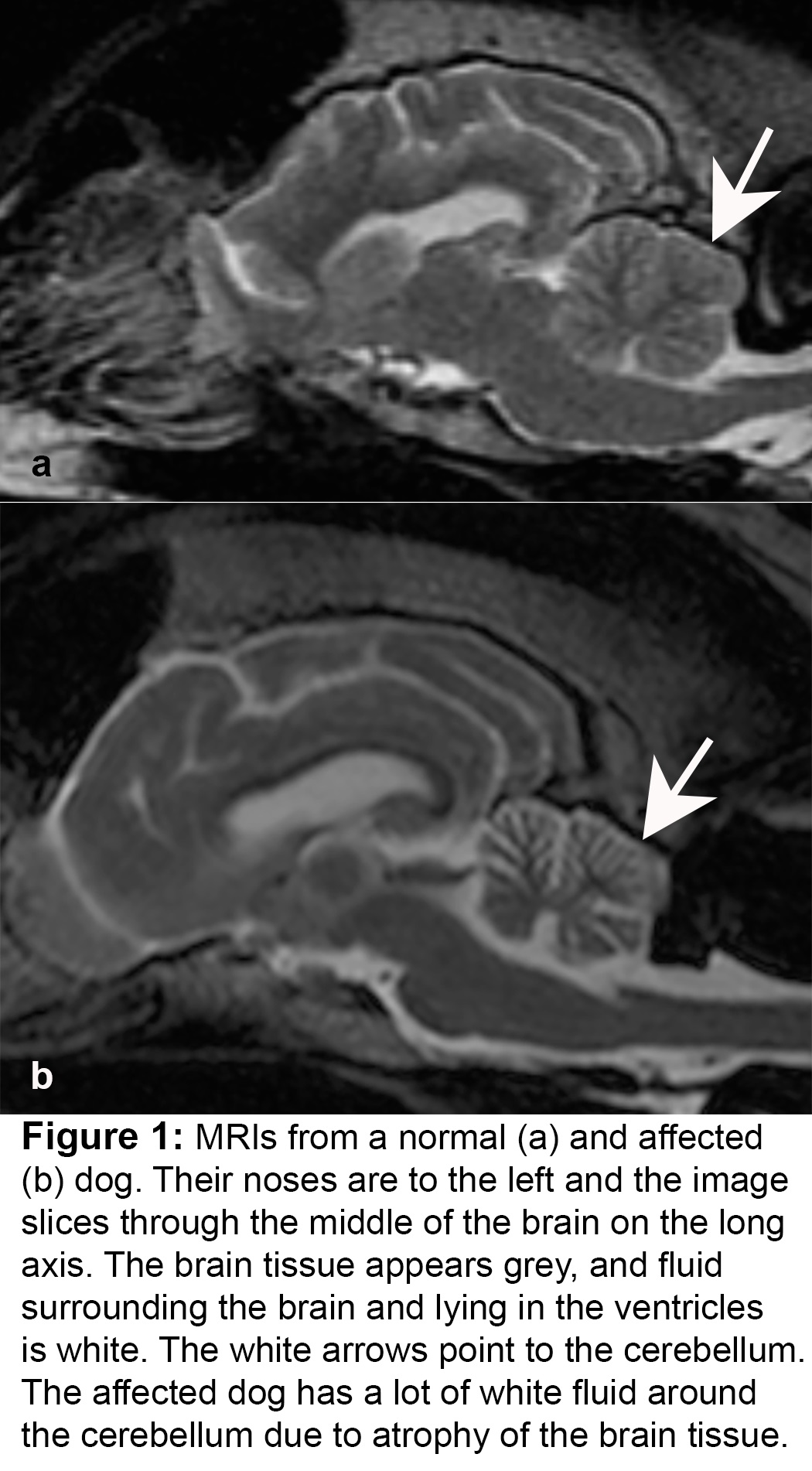
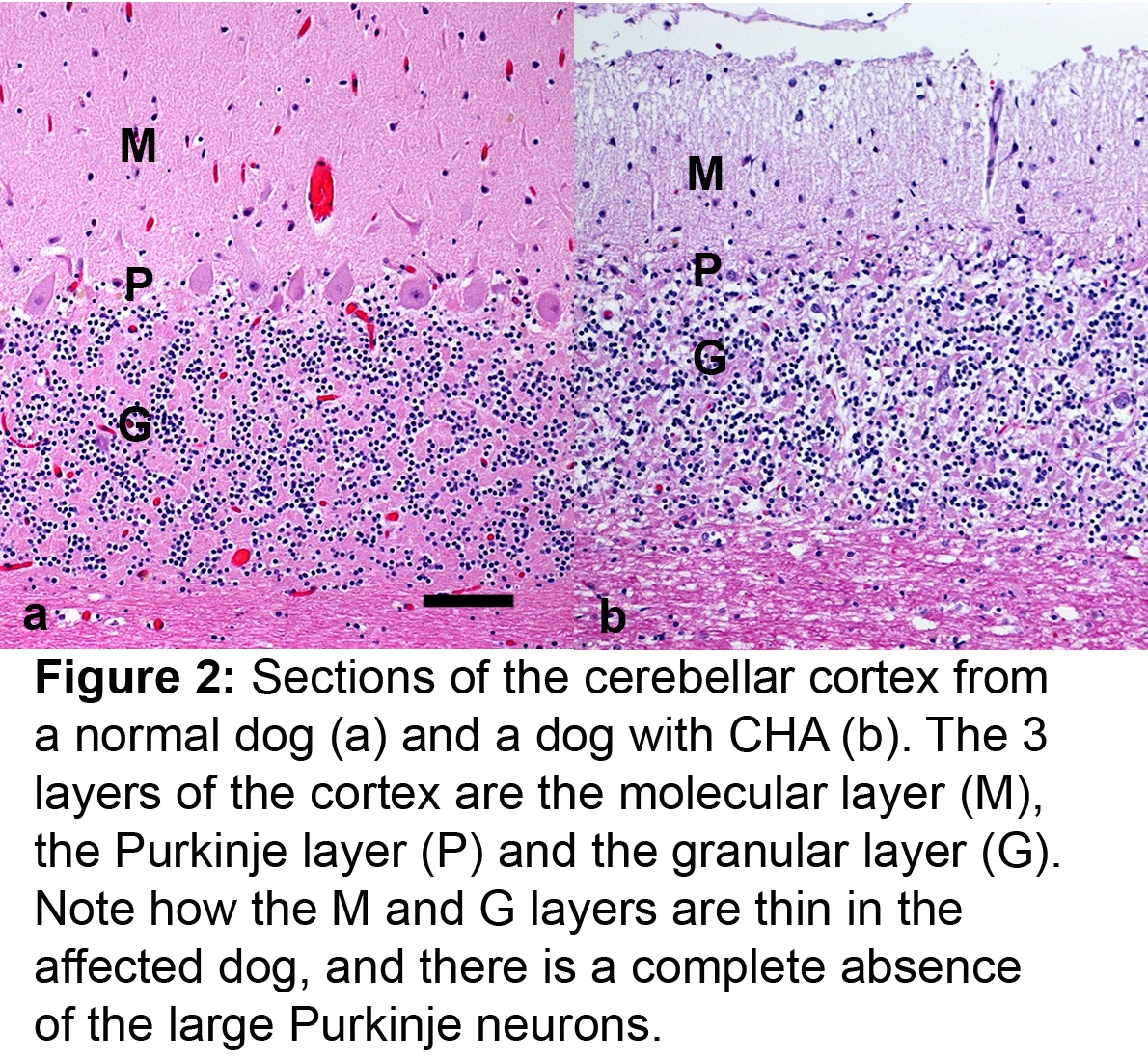
In the 1970s, an inherited disease that caused progressive difficulty walking was recognized in Gordon Setters and the first scientific reports appeared in the early 1980s. Dogs started to show signs of lack of coordination in adolescence and early adulthood, typically between 6 months and 4 years of age. These signs progressed until the dogs were unable to walk. The disease, named cerebellar abiotrophy at the time, was studied in detail by high profile veterinary research groups, and shown to be the result of gradual death of cerebellar neurons, in particular, the Purkinje neurons (figure 1 and 2). The disease is inherited as an autosomal recessive trait meaning that dogs had to have 2 copies of the underlying mutation in order to show signs; dogs with just one copy appear normal but can pass on the mutation, and so are called carriers. Affected dogs have appeared at a low rate worldwide ever since then.
What is the cerebellum?
The cerebellum is a part of the brain that controls the ability to move in a smooth and coordinated fashion. When the cerebellum is damaged, dogs develop cerebellar ataxia (ataxia simply means a lack of coordination; it can result from damage to many different parts of the nervous system): this is characterized by a high stepping gait, a wide based stance and exaggerated side to side swaying of the trunk (aka truncal ataxia). In addition, affected animals may show intention tremors (their head tremors from side to side when they fix their gaze on something – this is exaggerated by excitement and goes away when they are quiet), and nystagmus (flicking of the eyes from sided to side, up and down or round in circles).
Terminology – what do the names mean?
Neurodegenerative disease: This term includes all diseases in which neurons gradually die over time. Common examples in people include Alzheimer’s disease and Parkinson’s disease.
Cerebellar abiotrophy (CA): is a name given to inherited neurodegenerative diseases affecting the cerebellum in which animals are born with a normal cerebellum, and neurons die gradually over time. The term was coined to denote an intrinsic metabolic problem (a lack of trophic support), but now we know that these diseases can be caused by a wide range of defects, such as abnormal structural proteins, so the term is now used less commonly.
Hereditary ataxia: This term is an umbrella term that includes all inherited neurodegenerative diseases that cause progressive and intermittent ataxia. It is commonly used in humans, and was used by Linda Cork’s group to describe the disease in Gordon Setters (although, to be exact, they called it Canine Inherited Ataxia at the time), hence the acronym CHA – Canine Hereditary Ataxia. It is the term we have used in our publication. This term aligns well with the terminology used to describe people with similar diseases – indeed, when considering neurodegenerative diseases, Hereditary Ataxia is the third most common cause of difficulty walking after Huntington’s and Parkinson’s diseases in people.
Cerebellar Cortical Degeneration (CCD) and Cerebellar Degeneration (CD): can also be used to describe many neurodegenerative diseases of the cerebellum. The names are an accurate description of the changes found on pathology, with the term cortical included if the degeneration primarily involves the outer layers (the cortex) of the cerebellum. It avoids the implication of a problem with metabolism that comes with the name cerebellar abiotrophy.
Cerebellar ataxia: This simply means a lack of coordination due to cerebellar disease, in other words it describes a sign that can be common to any disease causing cerebellar damage. However, it has been picked up by some breed societies to mean cerebellar abiotrophy and this can cause confusion between dog owners and veterinarians.
Neuronal ceroid lipofuschinosis (NCL): is classified as a lysosomal storage disease. These diseases are hereditary neurodegenerative conditions in which a mutation causes accumulation of products that are usually broken down by lysosomes (an important disposal system within cells), causing gradual death of neurons. There is a form in Gordon Setters that causes progressive loss of the ability to walk, but also causes blindness and behavioral changes.
So to summarize – the disease we are discussing in Gordon Setters can be called Cerebellar Abiotrophy, Canine Hereditary Ataxia or Cerebellar Cortical Degeneration – all are correct, but our preferred term (to keep in line with the human classification) is Canine Hereditary Ataxia of Gordon Setters. Gordon Setters do suffer from a form of NCL but it causes different neurologic signs, and finally, the term cerebellar ataxia is simply a description of the way affected dogs walk, not the name of a disease.
What do we know about Canine Hereditary Ataxia in Gordon Setters now?
We have studied the genetic mutations underlying neurodegenerative diseases in dogs, including Old English Sheepdogs and Gordon Setters. Old English Sheepdogs have a neurodegenerative disease that causes identical signs to the disease seen in Gordon Setters. We started by mapping the disease in Old English Sheepdogs to a particular region of the 4th chromosome, and then we studied the DNA sequence of that region in detail and identified a mutation in a gene that was associated with the disease. By this we mean that every affected dog had 2 copies of the mutated gene, and normal parents of affected dogs each had 1 copy of the gene. The gene, Rab24, codes a protein that likely assists in disposal of waste proteins and cell organelles within neurons. We then looked at the same region in Gordon Setters and found that affected dogs also had 2 copies of the same mutation proving the both breeds, although very distinct, shared the same mutation. This tells us that the mutation has been around for a long time and was probably inherited from ancestors of both breeds of dog. A full account of this work can be found at: http://journals.plos.org/plosgenetics/article?id=10.1371/journal.pgen.1003991
What does this mean?
This finding is exciting for several reasons. First and foremost, we can test for the mutation – our genetic testing service does offer a test – more details about the test below. This allows breeders to test their breeding stock and avoid breeding 2 carriers of the disease, thus allowing gradual elimination of the mutation from the breed.
Second, this was the first report of an autophagy gene (a gene related to a particular disposal pathway) being associated with a neurodegenerative disease. This finding will allow us to study how neurons die in neurodegenerative diseases (eg Alzheimer’s Disease) and develop new therapies for these devastating and common conditions that afflict us as we age.
How prevalent is the mutation in Gordon Setters?
When we identified the mutation, we looked at a cohort of 90 Gordon Setters within the breeding population of dogs. We found the mutated allele at a frequency of 22.2% in this study population that included dogs from the US and from Scandinavia. Since then we have tested an additional 275 dogs, again worldwide, and we have identified the mutated allele in an additional 46 dogs – so 16.7% of dogs tested since our research carry the abnormal allele. The vast majority of these dogs were just carriers with no signs of the disease, so testing provided important information for their owners when it came to making decisions about breeding. This mutation is not at a very high frequency within the breed and it should be possible to eliminate it altogether by testing before breeding. Once a breeder has eliminated it from their kennel, further testing of their dogs is not necessary as long as they are bred to confirmed clear dogs.
How good is the test?
So far, the test has accurately detected 100% of affected dogs, including dogs that did not show signs until a bit older than typically seen. These dogs had reached breeding age well before developing any signs, so the test results were important to the owners. Based on the data we have so far in Gordon Setters, the test has been 100% specific and sensitive. The test is recognized by OFFA. As this research is ongoing, there are still questions that can only be answered with further studies. It has yet to be determined with 100% certainty whether we are testing for the mutation that actually causes the disease or just one that is linked to the disease. In order to prove a mutation causes a disease, it is necessary to do a functional test of the mutation – in other words quantify what the mutation does on a cellular level. It is rather difficult to test the process this mutation may affect and we are currently investigating this further.
How do you test for the mutation?
All information about testing your dogs can be found here
http://www.ncstatevets.org/genetics
http://www.laboklin.co.uk/laboklin/showGeneticTest.jsp?testID=8449


~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Previous information (pre 2015)
Cerebellar Degeneration CD, also referred to as Cerebellar Cortical Abiotrophy CCA, or Cerebellar Ataxia CA, is a hereditary neurological disease in the Gordon Setter breed. It is a slowly progressive neuromuscular incoordination caused by a simple autosomal recessive gene. Clinical signs can be recognized between six months to four years of age. Pathology studies performed in the 1970s show the age of onset closer to six months; but with mild clinical signs affected dogs may not be identified until later in life.
Clinical signs include: poor balance, frequent stumbling, a wide-based stance, head and/or body tremors, and a high-stepping gait. Affected dogs have normal mental alertness. Most affected dogs have a normal life expectancy and pass away from unrelated causes. There is no treatment or cure. CD is not related to the lethal metabolic encephalopathy (DUNG’d) seen in 3 to 6 week old Gordon Setters.
Drs. Alexander de Lahunta, Linda Cork, and Steven Steinberg identified the autosomal recessive mode of inheritance of CD in the breed and published its clinical description in several articles in the early 1980s. With this mode of inheritance, both parents must be carriers of the mutated gene to produce affected offspring. Approximately one-quarter of offspring from such matings are expected to be affected, but statistical chance can cause none to several affected dogs in a litter.
In 2012, Dr. Natasha Olby at North Carolina State University identified the mutated gene. A cheek swab test is available that will determine your dog’s genetic status as normal, carrier, or affected for CD. The test is available for any Gordon Setter worldwide through North Carolina State University (http://www.cvm.ncsu.edu/vhc/csds/vcgl/). It can be run at any age and costs $51(US) per test.
Gordon Setters affected with CD have been identified since at least the 1960s in both conformation and field lines throughout the United States, Canada, Europe, and Australia. Pedigrees of affected and carrier Gordon Setters worldwide do not “connect” before generationally early ancestors of the breed. All confirmed affected Gordon Setters around the world have the same identified mutated gene causing CD. Dr. Olby also identified this mutation as the cause of CD in the Old English Sheepdog breed – showing a common ancestor as the original source of the mutation in both breeds. No other breeds have been identified with the same mutation to date.
Over the years, Gordon Setter breeders and owners have been surprised by a diagnosis of CD in their dogs due to the lack of known relatives with the disorder. Historically, these occurrences are followed by more affected dogs from related lines. The ancient ancestral origin of the mutated gene explains this occurrence. The mutated gene has been dispersed and propagated in the Gordon Setter breed since its origination. Now that there is an inexpensive and accurate genetic test for the mutated gene, ALL breeding stock should be tested.
As with all testable simple autosomal recessive genes, quality carrier dogs can be bred to quality normal-testing mates. This prevents CD affected dogs from being produced while preserving and maintaining other positive traits. Quality normal-testing offspring should replace the carrier parent for breeding. Carrier offspring should be selected against for breeding homes. In this way, you have eliminated the single mutated gene, without losing the quality traits of the line. A genetic test for a simple recessive disorder should not change who gets bred, only who they get bred to.
To assist breeders with health-conscious breeding, each dog’s results should be entered into the OFA Cerebellar Degeneration registry (http://www.offa.org/pdf/dnaapp_bw.pdf). The test results will be listed on the dog’s OFA page. The cost is $15 per dog, $30 for a litter of 3 or more, and a kennel rate of $7.50 per dog if 5 or more dogs are entered by the same owner (all in $US). If a dog is out of two DNA tested normal Gordon Setter parents, the OFA will provide Clear by Parentage (CBP) certification. In this way, generations of Gordon Setters do not have to be tested. CBP certification requires that both parents are CD tested and entered into the OFA registry, and that the parents and offspring have been DNA parentage certified (usually available through your national Kennel Club).
Cerebellar Degeneration is not the most frequent genetic disorder affecting the breed, but is the oldest documented simple inherited disorder in Gordon Setters. With the availability of this accurate and inexpensive genetic test Gordon Setters should never again be affected with Cerebellar Degeneration.
This article can be reprinted with permission from the author Jerold S Bell DVM, Tufts Cummings School of Veterinary Medicine, N. Grafton, MA USA jerold.bell@tufts.edu
Home About Me Care Of Your Gordon News Litters Rogues Gallery Art Works Items Of Interest Links
Memories Darcey Antti Zena Lacey Torie Link Finn Laurelhach UK Laurelhach Overseas